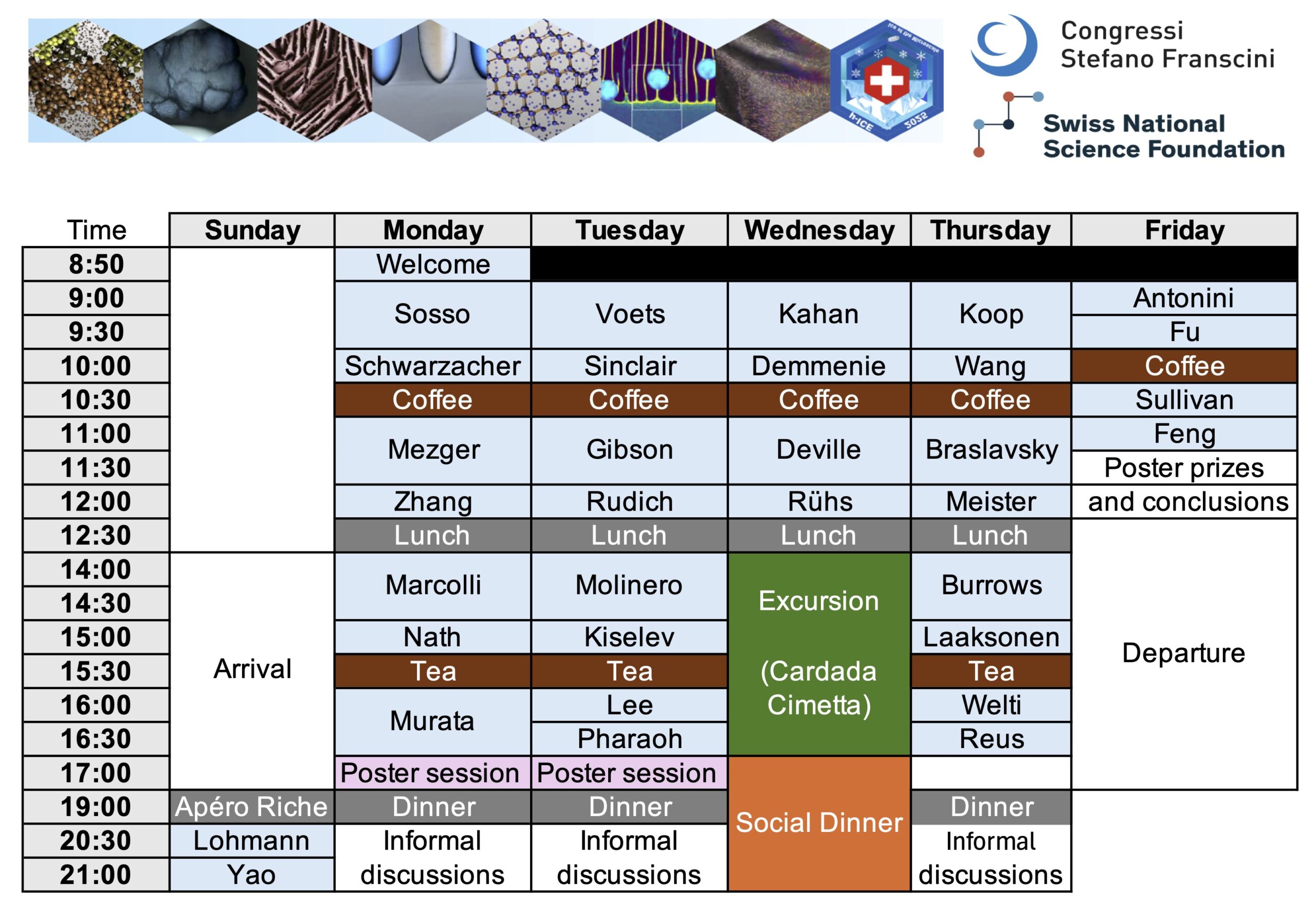
Downloadable programme
Click to download the programme along with the book of abstracts
This excursion will be to the Cardada-Cimetta. This is a mountain offering spectacular views over the Lake Maggiore. We will take a cable car/chair lift up to the top. Cardada is a great place for some hiking, but there are also many other activities available.
Sunday
20:30-21:00: Ulrike Lohmann "From climate to the microscale - the importance of ice nucleation"
Clouds are scientifically challenging to understand because their formation and dissipation require knowledge about both the large-scale meteorological environment as well as about the details of cloud droplet and ice crystal formation on the microscale. With the advancement of better in-situ and remote sensing instruments, unprecedented observations of clouds are now possible. We have exploited these advancements in our CLOUDLAB project, where we employed cloud seeding technology to better our understanding of mixed-phase cloud processes: by releasing silver iodide-containing particles from uncrewed aerial vehicles in supercooled low stratus clouds over the Swiss plateau, we were able to observe and measure downstream ice crystals in a controlled way. From these measurements, we quantifed the ice nucleated fraction and the ice crystal diffusional growth rates. Additional high-resolution modeling supported the experiments and provided insights for weather forecasts.
21:00-21:30: Yang Yao "Water crystallization under nanoconfinement"
Water, the most ubiquitous and essential liquid on Earth, is fundamental to the existence of life. In living cells, water is often found in crowded and confined environments, where its behaviour differs markedly from that in the bulk phase, particularly in terms of crystallization and dynamics. Understanding water under confinement is crucial for advancing fields such as cryopreservation, materials science, and biomolecular function at low temperatures.
In this talk, I will discuss our investigations into the crystallization and dynamics of water under both hard and soft confinement. First, I will present our findings on water confined within rigid media such as hollow silica spheres and mesoporous silica, which provide valuable insights into how geometric constraints influence ice nucleation mechanisms. I will then introduce our work on water confined within lipidic mesophases (LMPs), which represent a soft confining environment analogous to biological membranes. By employing a commercially available lipid (phytantriol), we successfully stabilized LMPs at subzero temperatures. Using a combination of differential scanning calorimetry and broadband dielectric spectroscopy, we identified two dynamically distinct fractions of confined liquid water: a slower fraction strongly bound to lipid head groups and a faster interstitial fraction that coexists with ice within the lamellar channels.
Monday
08:50-09:00: Welcome
Introduction to CSF and Monte Verita
09:00-10:00: Gabriele Sosso "First Steps Toward Understanding Ice Formation in Plants"
Understanding the formation of ice in plants is key to mitigating crop losses worldwide, particularly in light of the extreme weather oscillations arising from climate change. In contrast to ice formation in animal cells and tissues, however, our understanding of the microscopic details of ice formation within the plant cell wall remains limited. In this work, we take the first steps to address this gap through a synergistic approach combining plant biology experiments and molecular simulations. Specifically, we analyze the molecular-level details of the crosslinking of homogalacturonan (HG) pectin, comparing different microscopic mechanisms and exploring the effects of varying HG functionalisation patterns (deprotonated, protonated, methylesterified HG). We find that the extent and nature of HG crosslinking strongly influence the porosity of the cell wall, which we characterize by an experimental approach augmented by mathematical modelling. In turn, the topology of the HG network modulates the speed of ice propagation through plant tissue. We find that cold acclimation as well as supplementing extra Ca2+ to the plant (which enhances the HG cross-linking) lead to a similar increase in terms of frost resistance. In addition, our simulations suggest that while HG itself shows limited ice nucleating ability at the molecular scale, larger supramolecular assemblies or ordered networks may enhance ice nucleation activity to a significant extent. Interestingly, flooding the cell wall with water reverses the inhibitory effect of crosslinking on ice growth (due to the swelling of the HG network). Molecular dynamics simulations demonstrate that crosslinking controls pore size distributions and limits ice growth via the Gibbs-Thomson effect, providing a physical basis for freezing tolerance through cell wall remodeling and hydration control. These insights reveal how plants regulate ice formation and spread by tuning pectin architecture, offering promising targets for improving crop frost resistance.
10:00-10:30: Walther Schwarzacher "Ice Nucleation by DNA Origami"
In order to separate the influences of the multiple factors that contribute to ice nucleating efficiency, there is a need for model systems with chemical and physical structures that are well defined and easily varied. The exquisite control that it offers over physical geometry at the nanoscale makes DNA origami1 a particularly attractive model ice nucleating agent (INA). Here we compare ice nucleation by solutions of a rectangular DNA origami tile, formed by annealing a 2.6 kbase single-stranded DNA scaffold with ninety shorter ‘staple’ oligonucleotides, to ice nucleation when these components are mixed but not annealed. Isothermal measurements show that the molecular conformation has a dramatic effect on the ice nucleating efficiency. For an array of droplets containing annealed, well-folded tiles the freezing rate is constant with time, consistent with the presence of large numbers of identical INAs, whereas for unannealed DNA the freezing rate decreases with time, consistent with ice nucleation dominated by rare INAs, likely formed by molecular aggregation. A comparison of isothermal data and temperature ramp data shows an interesting phenomenon. Despite the freezing rate measured at low temperature being higher for annealed DNA origami samples than for a significant proportion of unannealed ones, in slow temperature-ramp measurements the latter mostly freeze at higher temperatures. By modelling the ice nucleating efficiency of the unannealed DNA using a log normal distribution, we show that our results are qualitatively as expected from classical nucleation theory.
11:00-12:00: Markus Mezger "Interfacial Premelting in Ice/Clay Nanocomposites"
At solid/ice interfaces, a premelting layer is formed at temperatures below the melting point of bulk water. This interfacial premelting layer in ice/clay nano composites was studied by quasi elastic neutron scattering and high energy X-ray diffraction. Using well defined and characterized ice/vermiculite, ice/kaolin, and ice/talc composite samples, this work bridges the gap between studies on single crystalline ice/solid model interfaces and naturally occurring soils and permafrost.
Below the melting point of bulk water, the formation of liquid water was observed. The liquid fraction is gradually increasing with temperature. Quantitative analysis of the molten fraction reveals differences in the deviation from Antonow’s rule relating the interfacial free energy between ice, water, and the clays. The translational diffusion constants of the confined premelting water is strongly reduced compared to super cooled bulk water. Adjacent to charged vermiculite the lowest water mobility was observed, followed by kaolin and the more hydrophobic talc. Results are explained by the intermolecular water interactions with different clay surfaces and interfacial segregation of the low-density liquid water (LDL) component.
12:00-12:30: Chao Zhang "Pore Ice Nucleation and Propagation via Water Nanofilms"
14:00-15:00: Claudia Marcolli "What are the nucleation sites of clay minerals"
Clay minerals are an important fraction of airborne dust. They are built of aluminosilicate sheets that are stacked together to form platy particles. The most common types of clay minerals, namely kaolinite, illite, and smectites, among which is montmorillonite, have all been shown to act as ice-nucleating particles (INPs) for cirrus and mixed-phase clouds despite their vastly different surface properties. While kaolinite is the only one to have an alumina surface, illite and smectite just have siloxane and edge surfaces. In contrast to kaolinite, illite and smectites both possess charge-balancing ions on their surface and between the sheets, but only smectites are swelling in water and aqueous solutions with a tendency to delaminate and even exfoliate. Despite these differences, there seem to be common structural elements that are responsible for their similar ice-nucleation (IN) activity. Yet, the surfaces and surface features required for their IN activity are still uncertain. In my presentation, I will show how experimental investigations can be used to elucidate the surface structures relevant to ice nucleation in clay minerals by targeted chemical and morphological modifications and by comparing the ice-nucleation activity of similar clay mineral samples. Specifically, I will show the influence of the charge-balancing ions on the IN activity of smectites, and the role of the stacking of sheets to platelets for the IN activity of smectites and kaolinites.
15:00-15:30: Saurabh Nath "Discovering Ice: Drops, Bubbles, Metastability "
Place a drop of water on an icy surface – it crystallizes at the point of contact, and a slow ice front steadily grows upwards. Place a bubble instead, and a burst of ice crystals erupts on the interface, far from where it touches the surface. Now, place a collection of dew drops – they start ‘talking’ to each other, freezing in succession, breaking the stochasticity of the nucleation process. The ordinary freezing of drops and bubbles hides a fascinating array of rich emergent phenomena, far from the ordinary. In this talk, through simple experiments and scaling arguments, we will delve into the nature and origins of these intriguing behaviors – often tied to the metastability of supercooled water. By exploring these phenomena, we will try to gain a deeper insight into the non-classical mechanisms of phase transitions in liquid water – be it in clouds or dew drops on a leaf…
16:00-17:00: Ken-ichiro Murata "Deliquescence revisited: insights from surface melting of ice"
Deliquescence is a phase transition in which solid substances absorb water vapor from the environment and spontaneously dissolve into an aqueous solution. A familiar example is the coarsening of table salt, which proceeds through deliquescence-induced capillary adhesion and subsequent recrystallization upon drying. Beyond its commonplace manifestations, deliquescence plays a significant role in global-scale phenomena. Sea salt aerosols, for instance, play a critical role in climate regulation via cloud formation [1]. Meanwhile salt weathering—driven by humidity-induced cycles of deliquescence and recrystallization (like a freeze-thaw cycle)—damages cultural heritage sites such as historic stone monuments [2]. With climate change causing increased atmospheric humidity, these negative impacts are expected to become more pronounced.
In this study, we revisit deliquescence by examining its underlying wetting behavior and thermodynamic stability across spatial and temporal scales, drawing particular attention to its striking resemblance to the surface melting of ice crystals [3]. Using advanced optical microscopy with a height resolution on the order of an angstrom (laser confocal microscopy combined with differential interference microscopy: LCM-DIM), we investigate the unique wetting behavior of deliquescent films, as well as their thermodynamic stability against crystallization. In situ observations using LCM-DIM revealed that deliquescent films do not grow continuously in thickness toward the deliquescence point, but rather emerge through nucleation in a first-order phase transition manner. Similar discontinuous behavior was also revealed in the quasi-liquid layers that we recently visualized [4]. We further found that deliquescent films exhibit two distinct thicknesses and that there exist transitions between these two wetting states (wetting transitions). From real-space analysis of the spreading dynamics, we succeeded in estimating the thickness of the thinner film to be approximately 3 nm.
17:00-19:00: Poster session
All posters will be in the Balint Room
Tuesday
09:00-10:00: Ilja Voets "Some like it cold: from the biophysical chemistry of antifreeze proteins to de novo designed ice-binders for chemical cryobiology"
The formation of ice (with)in cells, tissues, and other soft materials is often destructive beyond repair. Yet, there are many species (fish, bacteria, insects) that have developed effective coping strategies to mitigate freeze damage. These often revolve around ice-interactive materials that can manipulate ice nucleation and growth. My group studies how and why ice-interactive materials bind ice crystals and we relate their adsorption behavior to activity aiming to achieve a solid mechanistic understanding of how ice-interactive materials work and as a stepping stone towards the knowledge-based design of synthetic ice crystal growth modifiers for biomedical and other applications. In this talk, I will present highlights of recent work on ice-binding proteins and biomaterials, such as high-resolution imaging of individual ice-binders on ice using super-resolution microscopy and de novo designed ice-interactive proteins.
10:00-10:30: Brent Sinclair "Insect freeze tolerance: how important is structural change?"
We have known since the 18th Century that some insects can tolerate internal ice formation yet we still know remarkably little about the underlying mechanisms. I will briefly introduce insect freeze tolerance, and our understanding of the biochemical and molecular processes with which freeze tolerance is associated. I will then describe the currently accepted Extracellular Ice Formation (EIF) model, and the strength of evidence for and against it. In my lab, we use the spring field cricket Gryllus veletis as a model freeze tolerant insect; When freeze tolerant, G. veletis has suppressed metabolic rate and accumulates putative low molecular weight cryoprotectants (especially proline, myo-inositol, and trehalose). Recently, we have been exploring cellular- and tissue-scale remodeling in preparation for freezing, and how crickets maintain functioning mitochondria after being frozen; I will show how these observations fit the EIF model.
For further information, see coldbugs.com or contact bjs299@cornell.edu
11:00-12:00: Matthew Gibson "Chemical Cryobiology. Molecules and Materials to Probe Ice and Cryobiology"
Understanding and modulating temperature processes and ice formation/growth are crucial to a vast range of essential technologies for society: from di-icing windfarms for sustainable energy, to banking the current genetic diversity of crops, to efficient and safe distribution of advanced medicines and vaccines. Our approach to these challenges is the bottom-up discovery of new (macro)molecules to influence ice formation processes and /or modulate cryobiological outcomes.
Here I will update our progress on the discovery of ice binding (macro)molecules which spans low molecular weight ‘drug like’ compounds all the way to large nanomaterials. I will show how the control of ice formation/growth processes can directly impact cryobiological outcomes enabling unprecedented outcomes, including the storage of complex 2 and 3D cell models rapidly-deployable blood banking and protein/vaccine storage.
12:00-12:30: Yinon Rudich "Searching key mechanisms for ice nucleation by proteins"
Frost can form in nature at a few degrees below 0 °C. However, this process requires assembling water molecules into an ice-like structure to initiate freezing. Water ordering on this scale is mediated by ice nucleation proteins (INPs) found in common bacteria like Pseudomonas syringae and Pseudomonas borealis.
We tested the water ordering hypothesis to reveal how INPs trigger ice nucleation. We carried out double-blind freezing tests in the WISDON and BINARY apparatuses. Modeling of Pseudomonas borealis INP by AlphaFold predicted that the central domain is composed of 65 tandem 16-residue coils that form a β-solenoid with arrays of outward-pointing threonines and tyrosines, which may organize water molecules into an ice-like pattern. Deletion of sections of the 65 coils in INP expressed in E. coli Arctic Express led to a steady loss of ice nucleation activity until a sharper decline in activity was observed at ~25 coils and under. Mutating the water-organizing in parts of these coils decreased the ice nucleation ability even more. Insertion of a bulky domain had the same effect, indicating the importance of the continuity of the water-organizing repeats for the ice nucleation activity.
Simulations also showed that INPs are too small to organize enough water molecules for ice formation. We also demonstrate that a positively charged subdomain at the C-terminal end of the central β-solenoid is crucial for multimerization. Truncation, relocation, or change of the charge of this subdomain caused a substantial loss of ice nucleation ability. Cryo-electron tomography showed that the INP multimers form ~5 nm across and up to 200 nm long fibers. A model of these fibers as an overlapping series of antiparallel dimers can account for all their known properties. Overall, we provide important insights into ice nucleation by INPs.
14:00-15:00: Valeria Molinero "The Most Potent Snow Makers"
Several species of bacteria, fungi, lichen, and insects have evolved proteins that are potent ice nucleants. Bacteria and fungi are aerosolized and uplifted to the clouds, where they contribute to cloud glaciation and precipitation. Indeed, bacteria are so powerful ice nucleants that are routinely used for the synthetic production of snow. Despite the importance of biological ice nucleation for the survival and thriving of organisms and atmospheric processes, little is known about sequence and/or structure of the ice nucleating proteins, the mechanisms by which they promote water crystallization, and what makes them so outstanding. This presentation will discuss our approach to bridge the results of laboratory experiments, theory, numerical and molecular simulations, and artificial intelligence to elucidate the mode of action of biological ice nucleants. Our studies reveal that nature uses a common strategy among organisms in several kingdoms, “E pluribus unum” (out of many, one), to nucleate ice at temperatures close to the 0oC by assembly of ice-nucleating proteins into large functional aggregates.
15:00-15:30: Alexei Kiselev "Tailoring ice nucleating properties of alkali feldspar: hydrothermal alteration vs. micromachining"
Cloud ice plays a crucial role in Earth’s weather and climate system, influencing precipitation, cloud lifetime, and the radiation budget. Among mineral dust constituents, alkali feldspars – particularly perthitic varieties with complex microstructures – are the most ice-nucleation-active (INA). Their high IN activity is attributed to surface defects such as steps, pores, and cracks, which may expose (100) crystallographic facets recently identified as potential INA sites1, 2. While the link between IN activity and microstructural complexity is widely recognized, the molecular mechanism remains unclear.
Studies have shown significant variability in the IN activity of natural alkali feldspars, ranging from low IN activity in gem-quality Adularia to high INA in perthitic microcline. To investigate this, we simulate feldspar transformation by chemically and structurally modifying pristine, defect-free feldspars and measuring changes in their IN efficacy. Our previous work confirmed that chemically induced fracturing significantly enhances the initially low IN activity of K-rich alkali feldspar3. Here, we present our first results on the IN activity of hydrothermally altered K-rich alkali feldspar, using a method that closely mimics natural alteration of feldspar in aqueous environment of the Earth’s crust.
Additionally, we modify cleaved surfaces of pristine feldspar by micromachining cavities with one wall aligned to the (100) crystallographic orientation of feldspar, using Focused Ion Beam (FIB) milling. This enables direct testing of the hypothesis that (100) facets serve as primary high-temperature INA sites in alkali feldspar. IN activity is characterized using droplet freezing assays and ice nucleation experiments in an environmental SEM.
Complementing this study with in situ synchrotron X-ray diffraction, we aim to uncover the mechanism driving ice nucleation on natural alkali feldspars and the role of adsorbed water at the feldspar surface.
16:00-16:30: Du-Hyeong Lee "Porosity-dependent Crystallization Kinetics of Amorphous Solid Water Films"
Amorphous solid water (ASW) forms when water vapor is deposited onto cold surfaces and crystallizes as the temperature increases. However, certain details of the crystallization process, such as the precise nucleation sites, remain unclear. This presentation examines the crystallization kinetics of porous ASW (pASW) and compact ASW (cASW) to identify their nucleation sites. The porosity of ASW films was controlled by varying deposition temperatures and deposition angles. The extent of crystallization was monitored through desorption spectrometry. The results reveal that nucleation in pASW primarily occurs within the film interior, and cASW crystallization starts at the surface. Also, pASW crystallizes more rapidly than cASW, with lower activation energy (68.9 kJ∙mol−1 compared to 79.8 kJ∙mol−1 for cASW). These results enhance our understanding of the porosity effect on ASW crystallization and provide insights into the behavior of water molecules in cryogenic environments such as upper atmosphere and icy satellites.
16:30-17:00: Lian Pharaoh "The Ice Nucleation Mechanisms of Organic Hydroxylated Surface"
Organic hydroxylated ice nucleating substances (INSs) are thought to play an essential role in the climate by participating in cloud formation and modifying cloud properties. However, the mechanism by which these hydroxylated surfaces nucleate ice is not well understood. With the use of Molecular Dynamics (MD) simulations, the ice nucleation mechanism for fatty alcohol, fatty hydroxy ester and fatty acid monolayers can be investigated. Fatty alcohols exhibit an ‘odd-even’ trend in ice nucleation temperatures, in which alcohols with an odd number of carbon atoms within the alkyl chain will nucleate ice at warmer temperatures, than alcohols with an even number of carbon atoms. Our simulations show that the orientation of the C-O bond at the water interface must have a shallow enough angle for ice to nucleate, and alcohols with an odd number of carbon atoms tend to be more flexible, which allows them to reach the optimal C-O bond angle easier. The alcohols with an even number of carbon atoms are less flexible and require cooler temperatures to achieve the optimal C-O bond angle. Fatty hydroxy esters are the same as the fatty alcohols, with the exception of an ester group 9-10 carbon atoms away from the terminal hydroxy group at the water interface. Fatty hydroxy esters have poor 2D lattice matches to the lattice made by ice, but experimentally nucleate ice at warm temperatures. Our simulations show that the 2D lattice match of hydroxy esters improves as the temperature decreases because the associated carbon chain tilt angle decreases. Fatty acids are the most environmentally relevant and are poor ice nucleators. We show that fatty acids poor ice nucleation efficiency is attributed to the additional carbonyl oxygen atom at the water interface because it competes with the surface hydroxy groups to hydrogen bond to the water molecules
17:00-19:00: Poster session
All posters will be in the Balint Room
Wednesday
09:00-10:00: Tara Kahan "Microscopic Experiments, Macroscopic Implications: Toward Better Predictions of Chemistry in Ice"
Chemistry in snowpacks and at ice surfaces can dramatically affect atmospheric composition and the fate of atmospheric pollutants. Including snow and ice as reaction media in coupled chemistry-atmospheric models is challenging because we generally do not understand how reactions occur there. There is not a lot of research in this area, and existing results are often conflicting. We combine measurements of reaction kinetics in laboratory-prepared snow and ice with micro-spectroscopic investigations of the physicochemical properties of ice surfaces in the presence of environmentally-relevant solutes. This work shows that reaction environments can be very different at ice surfaces compared to in liquid aqueous solution, and provides mechanistic and kinetic insight that may help improve model parameterization.
10:00-10:30: Menno Demmenie "Ice is Not Covered by a Layer of Water"
The nature of the surface of ice has been subject of great controversy. Since Faraday’s proposal of an ever-present liquid layer on ice in the 1860s, nowadays often referred to as a “quasi-liquid-layer” or “liquid-like-layer”, its characteristics have remained a topic of contention. Depending on the experimental techniques employed or the sample size used in molecular dynamics simulations, the reported thickness of this putative layer spans up to three orders of magnitude, ranging from a few molecules to several micrometers [1,2].
We present an overview of our recent experimental investigations aimed at gaining deeper insights into the outermost layer of the ice crystal.
- Wetting Behavior: Sessile droplet experiments on ice reveal finite contact angles. Near the melting temperature, this angle cannot be explained solely by pinning due to crystallization. Instead, it must result from an energy balance between the surface energies.
- This partial wetting behavior is identified as the key factor influencing the formation of odd-shaped icicles grown from pure water. Interestingly the addition of a small amount of salt enhances the wettability, producing icicles with a characteristic cone shape and side ripples exhibiting a universal wavelength of about 1 centimeter [3].
- Scratch-Healing Dynamics: Indented microscale scratches in pristine ice surfaces were observed to spontaneously heal over time. Our findings show that sublimation from and condensation on the ice surface is the dominant mechanism driving this self-healing process; not a liquid-like flow [4].
These three sets of experiments challenge the traditional view of the outermost layer of ice as a “liquid-like” structure, as it shows that the behavior of the ice surface is inconsistent with that of a liquid.
11:00-12:00: Sylvain Deville "The Silent Architects of Freezing: How Solutes Shape Particle Behavior and Ice Growth"
Understanding the behavior of particles during freezing is essential across a range of fields, from cryobiology and materials science to climate studies and food preservation. In this talk, I will present how we use cryoconfocal microscopy to directly observe microscale dynamics in situ during ice growth. This technique offers a unique window into the spatial and temporal evolution of particles and solutes as they interact with a moving ice front. Focusing on the interplay between solute concentration, particles, and ice growth, I will show how these microscale processes shape macroscopic outcomes. Our observations challenge some of the assumptions of classical physical models and highlight their limitations in predicting real-world behaviors. By comparing model predictions with experimental findings, we reveal key mechanisms that are often overlooked, especially in complex or multicomponent systems.
12:00-12:30: Patrick Rühs "Addressing multicomponent complexity in freeze structuring of food colloids"
Freeze structuring (FS) is a valuable technique with exciting applications in food structure engineering. FS’s simple and scalable process allows precise control over pore morphology by manipulating ice crystal formation in suspensions via material and process parameters. However, food systems are inherently complex, heterogeneous, and polydisperse mixtures of polysaccharides, proteins, and fats. Unlike well-studied single-component systems, the interactions within multi-component mixtures during FS are challenging, limiting the broader adoption of FS for food systems.
In this work, we examine existing research on FS from various fields to identify the raw material characteristics and pre-processing strategies that influence structural outcomes in food systems, both for single components and for the holistic view of complex mixtures of these components. By enabling the direct structuring of complex mixtures without pre-purification, we aim to advance FS as a viable tool for sustainable food manufacturing. This approach underlines FS’s potential to improve resource efficiency and nutritional value, offering a path to innovative and scalable solutions for the food industry.
Thursday
09:00-10:00: Thomas Koop "Interactions of ice with proteins and polyols"
Ice nucleation is a critical process in the formation of ice clouds and the initiation of precipitation. A substantial body of research has previously been conducted on a range of heterogeneous ice nucleators, encompassing both laboratory experiments and fieldwork. The majority of these studies were traditionally concerned with particulate ice nucleators, but in recent years it has become apparent that ice nucleators of molecular origin can also catalyze ice nucleation, sometimes even very effectively. However, a comprehensive molecular understanding of their mechanisms of action remains elusive, underscoring the necessity for dedicated experiments towards that goal.
A comprehensive investigation was conducted into a range of naturally occurring and synthetic substances, some of which have atmospheric relevance. To this end, two experimental techniques were employed for the detection of droplet freezing. Firstly, the BINARY (Bielefeld Ice Nucleation ARraY) setup utilizing microliter-volume droplets, and secondly, the recently developed nanoBINARY (nanoliter Bielefeld Ice Nucleation ARraY) setup, which is a microfluidic apparatus employing nanoliter-volume droplets. The combination of these two devices enables the study of freezing processes over a broad temperature range, extending from the ice melting point down to the homogeneous ice nucleation limit of water droplets. The latter ability is an essential prerequisite for the study of molecular ice nucleators, which sometimes induce heterogeneous ice nucleation only at low temperatures in proximity to the homogeneous ice nucleation limit. In conjunction with the aforementioned ice nucleation experiments, the results of other experiments dedicated to the study of ice growth inhibition induced by molecular compounds adsorbed to the surfaces of existing ice crystals are presented. This objective is realized through the implementation of the sucrose-sandwich technique in conjunction with a subsequent kinetic analysis of ice recrystallisation, a process which we termed the Ice Recrystallization Rate INhibition Analysis (IRRINA) assay.
The presentation will comprise data on ice nucleation and ice growth inhibition induced by proteins as well as oligomers and polymers of natural and synthetic origin, including polyols and polysaccharides.
10:00-10:30: Jianjun Wang "Microscale Mechanisms of Ice Regulation in Cryopreservation"
Cryopreservation is a critical technology for the long-term storage of biological samples, including cells, tissues, and organs, and plays a pivotal role in biomedicine fields such as cell therapy, regenerative medicine, and organ transplantation. At the microscale, the high-water content of cells and tissues (70%–90%) presents a significant challenge: ice formation, both intracellularly and extracellularly, which can lead to mechanical damage, osmotic shock, and solute accumulation. The scientific challenge of cryopreservation lies in effectively inhibiting or controlling ice formation to mitigate these damages. Traditional cryopreservation strategies rely on cryoprotective agents (CPAs) such as dimethyl sulfoxide (DMSO), which inhibit ice formation by vitrifying water. While CPAs have achieved success in preserving cell suspensions, their cytotoxicity, and inefficiency in controlling ice formation limit their application to complex biological structures like tissues and organs. Nature offers a microscale solution through ice-binding proteins (IBPs), which regulate ice nucleation and growth by controlling the site, temperature, and morphology of ice crystals. Inspired by the mechanisms of IBPs, we have developed Ice Controlling Materials (ICMs), which mimic these natural processes [1]. Unlike conventional CPAs that prevent ice formation entirely, ICMs allow controlled ice crystal formation while precisely managing their size, shape, and nucleation site. This approach alleviates ice-related damage and enhances preservation outcomes. The development of ICMs has not only advanced cryopreservation technology but also deepened our understanding of the microscale mechanisms organisms use to protect themselves from freezing damage. By leveraging these materials, efficient and safe cryopreservation of tissues and organs is being realized [2], marking a transformative step in the field.
11:00-12:00: Ido Braslavsky "Ice-Binding Proteins as Modulators of Roughening Transitions and Fortifiers of Ice–Cellulose Composites"
12:00-12:30: Konrad Meister "How Proteins Control Ice Formation"
Ice nucleation by proteins plays a crucial role in atmospheric and ecological processes, influencing cloud formation, precipitation, and biological survival strategies. While theoretical studies suggest that large molecular size and strong ice-binding affinity are key factors in potent ice nucleation, experimental validation has been limited. Here, we integrate ice nucleation measurements, physicochemical characterization, numerical modeling, and nucleation theory to provide direct evidence that efficient ice nucleation depends on two primary factors: (1) large protein size, often achieved through the functional aggregation of smaller units, and (2) a strong affinity for ice. We elucidate the hierarchical assembly mechanism of bacterial ice-nucleating proteins (INPs), which form multimers that enhance their nucleation efficiency. The environmental sensitivity of the bacterial INP assembly to pH and ionic content provides a pathway for optimizing their performance in controlled freezing applications.
Beyond bacterial systems, we identify fungal ice-nucleating proteins as smaller extracellular proteins capable of assembling into functional aggregates. This discovery highlights a similar strategy across biological kingdoms, where protein aggregation enables ice formation at relatively warm subzero temperatures. Our findings establish a framework for identifying and engineering potent ice-nucleating proteins, with implications for atmospheric science, cryopreservation, and industrial freezing technologies.
14:00-15:00: Susannah Burrows "TBA"
TBA
15:00-15:30: Ari Laaksonen "Ice nucleation in adsorbed water"
Heterogeneous vapor-liquid nucleation of water occurs when a sufficiently large number of water molecules come together at a surface, forming a cluster that does not decay but initiates condensation. In the classical view, deposition ice nucleation occurs analogously to vapor-liquid nucleation, with the difference that the water molecules form not only a cluster but a hexagonal crystal. The requirement of the crystalline structure of the cluster obviously greatly decreases the probability of nucleation, and it seems likely that an intermediate liquid phase would facilitate ice nucleation. In the pore condensation and freezing mechanism (Marcolli, 2014), the intermediate liquid phase is capillary condensed water. However, deposition ice nucleation occurs on different nonporous ice nuclei as well, suggesting that multilayer adsorbed water may in these cases act as the intermediate phase. We have developed a theoretical framework to account for the homogeneous and heterogeneous freezing of adsorbed water films and droplets. Water vapor adsorption is described using the FHH adsorption model, which is commonly used in studies of CCN activation of insoluble particles (Sorjamaa and Laaksonen, 2007), and the freezing rates are calculated using the classical nucleation theory. The theory predicts very diverse temperature dependencies of the critical supersaturation that are determined by the adsorption parameters and ice and water contact angles of the ice-nucleating materials, and the requirement that the adsorption layer thickness, or adsorbed droplet size, must be large enough to accommodate the critical ice cluster. The predictions agree remarkably well with laboratory experiments.
16:00-16:30: André Welti "Time dependence of deposition ice nucleation"
To investigate the fundamental mechanism of ice nucleation below water saturation through its time dependence, experiments on insoluble, non-porous particles were conducted using the modified Spectrometer for Ice Nuclei (SPIN) described in Welti et al. (2020). SPIN is a continuous flow diffusion chamber in which test particles are exposed to constant temperature and humidity conditions for a residence time of 8-12 seconds. Formed ice crystal in the size range of 0.4-15 µm are detected at the exit of the SPIN chamber based on the intensity of single particle light scattering.
To investigate the time dependence, the ice crystal size distribution is inverted to obtain ice crystal growth times, and by subtracting the ice crystal growth times from the residence time in the SPIN chamber, the occurrence of nucleation events as function of time is reconstructed.
Within the limited residence time of the experiment, we observe a sigmoidal shaped time dependence of nucleation events indicating a time-dependent nucleation rate.
This is in contrast to a logarithmic curve shape, as expected from classical nucleation theory, which assumes a constant, time-independent nucleation rate under fixed environmental conditions.
Excluding experimental errors as cause of the observed time dependence, plausible mechanisms to explain the data are: non-steady state nucleation, a time-dependent adsorption of water molecules which affects the ice nucleation rate (Fletcher, 1963), or a time-lag due to the arrangement of condensate molecules into an orderly lattice (Vonnegut, 1949).
The observed strong time dependence constrains the possible mechanism of ice nucleation below water saturation, improves the interpretation of experimental observations, and can guide the development of ice nucleation theory.
16:30-17:00: Thomas Reus "Pinning of ice-water interfaces by ice-binding proteins explains ice-recrystallisation inhibition"
The suppression of ice recrystallization by ice-binding proteins (IBPs) plays a vital role in survival strategies of freeze-tolerant organisms. While the mechanisms behind ice-recrystallization inhibition (IRI) have remained elusive, the thermal hysteresis (TH) effect, a survival strategy of freeze-avoiding organisms, has a well-established underlying physical mechanism of advancing ice plane pinning by IBPs. This study formulates and substantiates a comprehensive physical model for IRI by IBPs that ties together the physical foundations of TH and IRI. We adopt the well-established framework of diffusion-limited Ostwald ripening as the fundamental basis of ice recrystallization and incorporate transient pinning by IBPs on the ice-crystal surface. Mathematical modeling and numerical simulations reveal that transient pinning homogenizes surface curvature across ice crystals, demonstrating effective IRI at a remarkably low density of ice-bound proteins. We discuss the validity of our model in view of related theory and experimental observations, quantify the impact of ice-IBP reversibility and binding kinetics, and provide a range of testable predictions on the dependence of IRI activity on experimentally accessible parameters. This research contributes to our fundamental understanding of cryoprotection by ice-binding proteins, which can facilitate further exploration and development of ice binding agents and their use in cryopreservation applications.
Friday
09:00-09:30: Carlo Antonini "Discontinuity-enhanced icephobicity as a strategy for reduced ice adhesion on surfaces"
Passive low ice-adhesion surfaces are frequently composed of soft materials; however, soft materials potentially present durability issues, which could be overcome by fabricating composite surfaces with patterned rigid and soft areas. Here we propose the innovative concept of discontinuity-enhanced icephobic surfaces, demonstrating that the stress concentration at the edge between rigid and soft areas facilitates ice detachment.
Complementing experimental tests with numerical simulations, it was found that on a composite surface containing rigid and soft areas, stress is concentrated at the edge between the two, i.e. at the discontinuity line, rather than all over the soft or rigid areas. As a result, ice detachment is promoted: the crack occurs first at the discontinuity line, propagates on rigid and then on soft areas. Moreover, it was demonstrated that an increase in discontinuities promotes crack initiation and leads to a reduction of ice adhesion. Remarkably, an unexpected non-unidirectional crack propagation was observed for the first time and elucidated.
09:30-10:00: Ruby Fu "Freezing flow in porous ice"
Climate warming is altering hydrological processes in the cryosphere, yet these changes remain poorly understood. This study advances our understanding of fluid flow through subfreezing porous materials like snow, firn, and permafrost. Such flow involves complex interactions between interfacial flow, phase change, and thermal transfer, leading to diverse non-equilibrium phenomena from pore to field scales. We present ongoing numerical and experimental investigations into unsaturated water flow in porous ice.
At the cm-to-m scale, we employ a thermodynamic nonequilibrium infiltration model to examine refrozen structures formed during gravity-driven water infiltration in subfreezing porous media. We identify two key freezing-induced mechanisms that slow infiltration. First, a portion of infiltrating water freezes, reducing the effective infiltration rate, which can be quantified using the freezing Damköhler number. Second, secondary fingers—new flow paths forming between primary channels—decrease flow channelization, weakening infiltration efficiency through flow field homogenization.
At the mm-to-cm scale, we explore water imbibition into a subfreezing Hele-Shaw cell through experiments and modeling. Water at 0°C is injected at a constant flow rate into a radial Hele-Shaw cell cooled by a thermal-controlled aluminum block. By varying the cell gap thickness and flow rate, we analyze changes in solidification dynamics and flow. To model this process, we develop a gap-averaged two-phase Hele-Shaw flow model coupled with freezing dynamics. Finally, we compare preliminary experimental results with the model.
10:30-11:00: Sylvia Sullivan "Sensitivity of atmospheric ice cloud radiative effects to ice crystal complexity"
In-situ measurements indicate that atmospheric ice crystals are quite complex, with an array of different morphologies and degrees of surface roughness, aggregation, or attrition (Järvinen et al., 2018). We perform a series of single-column radiative transfer calculations to quantify the impact of this ice crystal complexity on the radiative heating from ice clouds in the atmosphere. Inclusion of complexity reduces this cloud-radiative heating (CRH), while temperature dependence of optical properties enhances the heating, especially for optically thin ice clouds at high altitudes. We then compare these findings from the idealized setup to optical sensitivities in a full complexity storm-resolving model, the Icosahedral Non-hydrostatic (ICON) model. ICON simulations are run for several different descriptions of ice optical properties in the atmosphere. In these more realistic simulations, the daily cycle of CRH is muted and the outgoing infrared radiation enhanced by including ice crystal complexity or temperature dependence in the optical properties. Changes in cloud-radiative heating of even a few degrees Kelvin per day have important implications for atmospheric circulations and stability. We finish with a brief overview of an effort at The University of Arizona to design the CHanneled Infrared Polarimeter (CHIRP), an infrared polarimeter that would provide observational data on ice crystal complexity using polarization measurements in 8-11.5 µm wavelength band.
11:00-11:30: Yanxia Feng "Characterizing hydrogel behavior under compression with gel-freezing osmometry"
Hydrogels are particularly versatile materials that are widely found in both Nature and industry. One key reason for this versatility is their high-water content, which lets them dramatically change their volume and many of their mechanical properties — often by orders of magnitude — as they swell and dry out. Currently, we lack techniques that can precisely characterize how these properties change with water content. To overcome this challenge, here we develop Gel-Freezing Osmometry (GelFrO): an extension of freezing-point osmometry. We show how GelFrO can measure a hydrogel’s mechanical response to compression and shrinkage in response to an applied osmotic pressure, while only using small, 100mL samples. Because the technique allows measurement of properties over an unusually wide range of water contents, it allows us to accurately test theoretical predictions. We find simple, power-law behavior for both mechanical and osmotic responses, while these are not well-captured by classical Flory-Huggins theory. We interpret this power-law behavior as a hallmark of a microscopic fractal structure of the gel’s polymer network and propose a simple way to connect the gel’s fractal dimension to its mechanical and osmotic properties. This connection is supported by observations of hydrogel microstructures using small-angle x-ray scattering. Finally, our results motivate simplifications to common models for hydrogel mechanics, and we propose an updated hydrogel constitutive model.